12+ Chapter 8 Covalent Bonding Answer Key
Ester is obtained by an. Formally a string is a finite ordered sequence of characters such as letters digits or spaces.

Covalent Compounds Covalent Bond Properties Examples With Videos
Web Starchs chemical formula is represented as eqC_6H_12O_5n eq where n represents the number of glucose molecules covalently bonded together.

. Or a chemical reaction resulting in the formation of at least one ester product. NCERT Solutions for Class 10 Maths Chapter 13. Web Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.
To perform well in the Class 12 Chemistry board exam students must understand Chapter 1 thoroughly. The simplest organic carbon molecule is methane CH 4 in which four hydrogen atoms bind to a carbon atom. The Chapter 15 NCERT Solutions for Class 12 has been solved by expert teachers as per the latest CBSE guidelines.
Figure 86 Electronegativity Difference Diagram. The sharing of electrons helps to get the octet configuration to both bonded atoms. UPSC Prelims 2022 Answer Key.
Covalent bonds can be single double or triple covalent bond. Web CONNECT WITH US. Web Recall that the electronegativity difference can be used to determine the polarity of a substance.
If the atoms that form a covalent bond are identical as in H 2 Cl 2 and other diatomic molecules then the electrons in the bond must be shared equallyWe refer to this as a pure covalent bondElectrons shared in pure covalent bonds have an equal probability of being near each nucleus. We place three lone pairs of electrons around each F atom accounting for 12 electrons and giving each F. Figure 212 Carbon can form four covalent bonds to create an organic molecule.
The chemical reaction that takes place during the formation of the ester is called esterification. An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial. 8 6 7 50.
NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure are provided in this article for CBSE studentsComprehensive answers to every question listed in the NCERT Class 11 Chemistry textbook can be. NCERT Solutions for Class 10 Maths Chapter 13. IAS Coaching Delhi.
Download the PDF for free and revise these important questions for CBSE exam 2022-23. Norton Company Inc. Web Get chapter-wise important questions for CBSE Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties with answers on Vedantu.
Web We would like to show you a description here but the site wont allow us. The empty string is the special case where the sequence has length zero so there are no symbols in the string. Figure 128 A contour map showing stratospheric ozone concentration and the ozone hole that occurs over.
Web a The bond formed between two atoms by mutual sharing of one or more pair of electrons os called as covalent bonding. Web Heterolytic fission is favored when bonding atoms have electronegativity differences and the presence of polar solvents at low temperatures. Esterification is the process of combining an organic acid RCOOH with an alcohol ROH to form an ester RCOOR and water.
All atoms tend to complete the octet configuration that provides stability to them. Students of Class 11 who are looking to give their best for the upcoming Class 11 first term exams and competitive exams need to be accustomed to NCERT Solutions for Class 11 Chemistry Chapter 12With the help of these NCERT. In homolytic fission a covalent bond breaks in such a way that each of the bonded atoms gets one of the shared electrons.
One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Modification of work by the Italian voiceFlickr. Web NCERT Solutions for Class 10 Maths Chapter 12.
Web Covalent bonds are formed by equal sharing of electrons between bonded atoms. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. For example ethane has 2 atoms of carbon and 6 atoms of hydrogen.
Web What is Esterification. Web Join an activity with your class and find or create your own quizzes and flashcards. Web These solutions will help you to take chapter notes polymers too.
Class 12 Chemistry Chapter 15 Polymers Solutions are given here for better understanding and clarification of the chapter. Web NCERT Solutions Class 11 Chemistry Chemical Bonding and Molecular Structure Free PDF Download. B i Bond formed between two Cl atoms.
UPSC Prelims 2022 Answer Key. Web Class 12 Chapter 1 The Solid State is an essential chapter that helps you to understand the basics of Chemistry as it focuses on the State of Matter. Web NCERT Solutions for Chemistry Class 11 Chapter 12 Organic Chemistry Some Basic Principles and Techniques.
8 2 7 22 XeF 6. NCERT Solutions for Class 10 Maths Chapter 11. NCERT Solutions for Class 10 Maths Chapter 14.
A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms attain their nearest inert gas configuration. Plants create starches in two forms. Web 8 Advanced Theories of Covalent Bonding.
NCERT Solutions for Class 10 Maths Chapter 12. Distribute the remaining electrons. The state of matter forms the fundamentals for many chapters later on.
Ii Bond formed between a hydrogen atom and chlorine atom. Draw a skeleton joining the atoms by single bonds. Covalent bonds are usually formed between two non-metals.
The simplest carbon molecule is. How many electrons are shared in each. Web Carbon contains four electrons in its outer shell.
Modification of work by vxlaFlickr. Web What is an Ionic Bond. Therefore it can form four covalent bonds with other atoms or molecules.
Students studying in Class 12 might be. 81 Valence Bond Theory. It can also infer the bonding structure of one molecule in.
Web NCERT Solutions for Class 10 Maths Chapter 8. The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond. 82 Hybrid Atomic Orbitals.
Xenon will be the central atom because fluorine cannot be a central atom. Typically an ionic bond has an electronegativity difference of 18 or above whereas a polar covalent bond is between 04 to 18 and a nonpolar covalent bond is 04 or below. Web Water is the chemical substance with chemical formula H 2 O.
They can be polar or.

Experimental And Multiscale Quantum Mechanics Modeling Of The Mechanical Properties Of Pvc Graphene Nanocomposite Amin Hamed Mashhadzadeh Abdolhossein Fereidoon Morteza Ghorbanzadeh Ahangari 2020
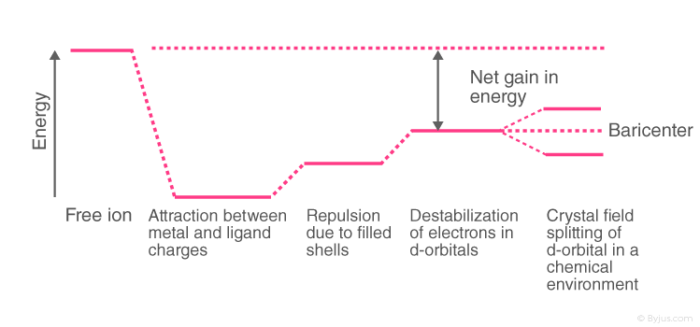
Crystal Field Theory Cft Detailed Explanation With Examples Videos

Chemistry The Science In Context Volume I And Ii 4th Edition Gilbert
Chapter 8 Redox Reactions Ppt For Class 11 Cbse

Chapter 3 Molecules Compounds And Chemical Equations Genevieve Wysocki Chapter 3 Molecules Studocu

Chapter 8 Covalent Bonding Answer Key Pdf Fill Online Printable Fillable Blank Pdffiller
Why Is Bcl3 An Electron Deficient Compound And Why Does It Have A Strong Tendency To Gain An Additional Pair Of Electrons By Reacting With Species With A Lone Pair Of Electrons
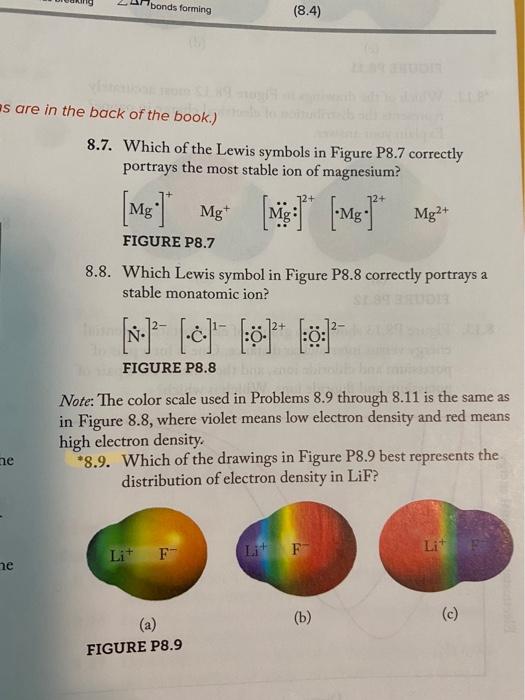
Solved Bonds Forming 8 4 Es Are In The Back Of The Book Chegg Com

Covalent Bonding Review Sheet 2012 Answer Key Pdf

Chapter 8 Covalent Bonding Flashcards Quizlet

Covalent Bonding Worksheet Colina Middle School

Class 11 Chemistry Chapter 4 Solutions Archives Esaral
Why Is Bcl3 An Electron Deficient Compound And Why Does It Have A Strong Tendency To Gain An Additional Pair Of Electrons By Reacting With Species With A Lone Pair Of Electrons
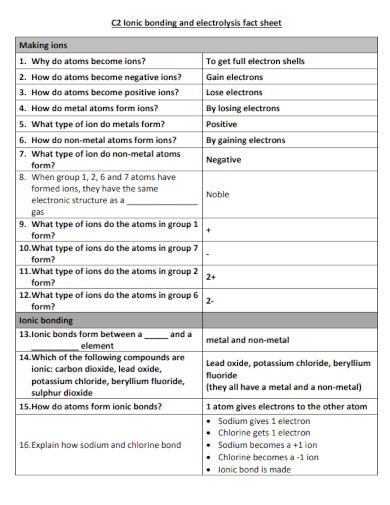
Ionic Bond Examples Pdf Examples
![]()
Nearpod

Us6951962b2 Oil Grease And Water Sizing Agent For Treatment Of Cellulosics Google Patents
![]()
Nearpod